Summary:
Click image to enlarge
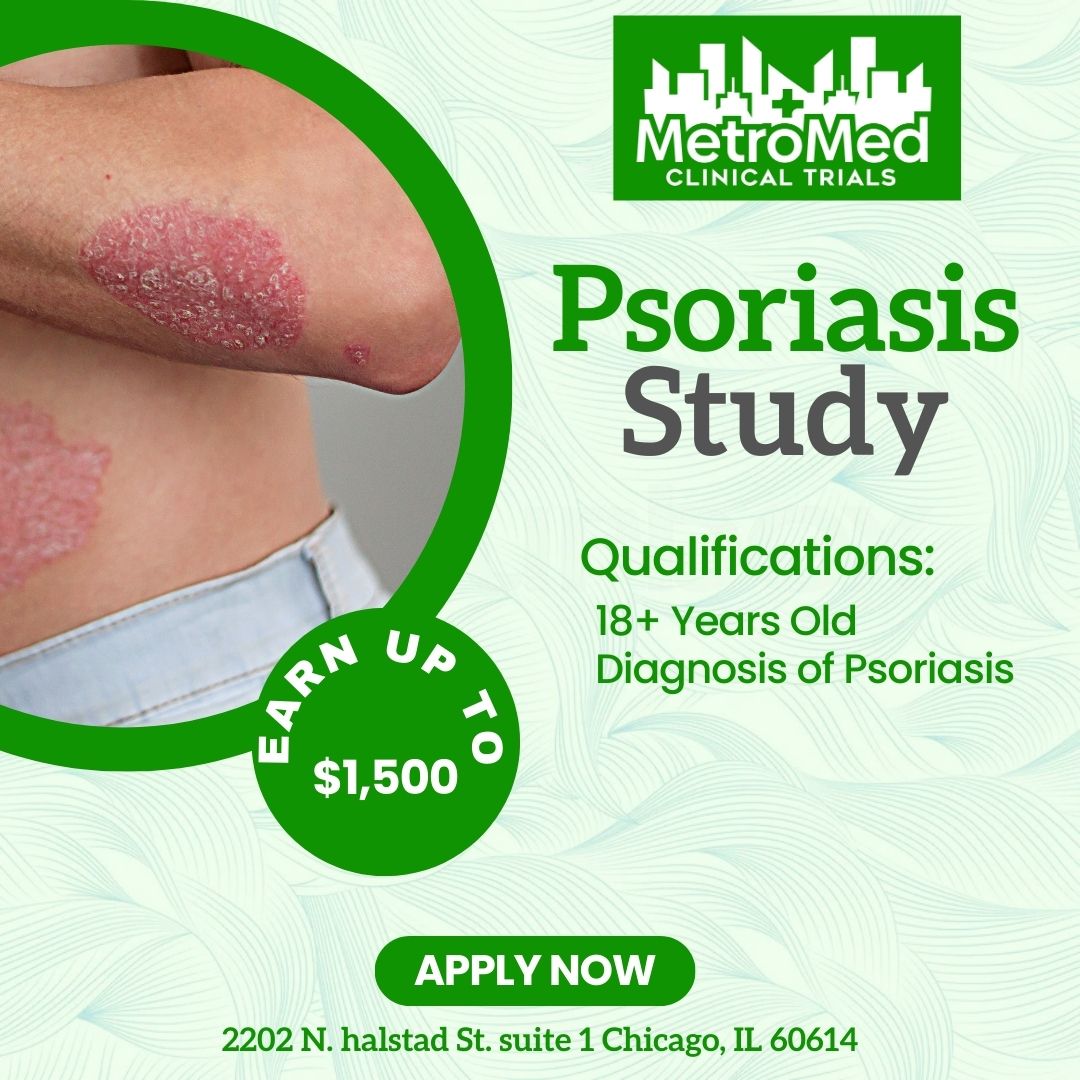
This clinical study is testing an investigational drug called ESK-001 for the possible treatment of plaque psoriasis. “Investigational” means the study drug is approved for use in clinical research but not yet approved to be prescribed to treat the condition. ESK-001 works by blocking certain proteins in your immune system, which may help to reduce plaque psoriasis symptoms.
Qualified Participants Must:
• At least 18 years of age
•
Diagnosed with plaque psoriasis at least 6 months ago
•
Have plaques covering at least 10% of your body
Qualified Participants May Receive:
- Compensation: Earn UP to $1,500
- Study-related treatment at no cost